TPN-101 is the first PSP treatment to reduce NfL and IL-6 levels, key biomarkers of neurodegeneration and neuroinflammation in PSP
Participants treated with TPN-101 for the entire 48-week study in PSP showed a stabilization of their clinical symptoms as measured by the PSPRS
In the Phase 2 study of C9orf72-related ALS/FTD, TPN-101 showed effects on key biomarkers of neurodegeneration, neuroinflammation and microglial activation, including NfL, Tau, UCHL1, YKL-40 and osteopontin
TPN-101 also showed a clinical effect on Vital Capacity, an objective respiratory measure that correlates with mortality in patients with ALS
Transposon plans to advance TPN-101 to a Phase 3 registration study for PSP and is expanding the development of TPN-101 into Alzheimer’s disease
Transposon Therapeutics, a biotechnology company developing a platform of novel, orally administered therapies for the treatment of neurodegenerative and aging-related diseases including Alzheimer’s disease, today announced final results from its Phase 2 study of TPN-101 for the treatment of progressive supranuclear palsy (PSP). The company also announced interim results from its Phase 2 study of TPN-101 in patients with amyotrophic lateral sclerosis (ALS) and/or frontotemporal dementia (FTD) related to hexanucleotide repeat expansion in the C9orf72 gene (C9orf72-related ALS/FTD). Transposon will present final results from its Phase 2 study in PSP in a poster presentation at the hybrid AD/PD™ 2024: 18th International Conference on Alzheimer’s and Parkinson’s Diseases. The meeting will take place online and in Lisbon, Portugal, from March 5-9, 2024.
Final Phase 2 PSP Study Results
Final 48-week results from the Phase 2 study in PSP confirm and extend the 24-week interim results that showed TPN-101 is the first treatment for PSP to reduce levels of neurofilament light chain (NfL), a key biomarker of neurodegeneration in tauopathies such as PSP and Alzheimer’s disease. Participants treated with placebo from weeks 1 to 24 and then switched to 400 mg TPN-101 at week 24 experienced a reduction of NfL in the cerebrospinal fluid (CSF) from weeks 24 to 48 that was similar to that observed in the group of participants receiving the 400 mg dose from weeks 1 to 24. Participants treated with 400 mg TPN-101 for the entire 48-week treatment period showed no increase of NfL levels in the CSF from weeks 1 to 48. In contrast, NfL levels in the CSF are reported to increase by 9-18% annually in natural history studies of PSP.
The 48-week results also showed further dose-related reductions in interleukin 6 (IL-6) cytokine levels, as well as reductions in levels of osteopontin. IL-6 is a biomarker of neuroinflammation that is elevated in PSP and correlates with disease progression and severity. Osteopontin levels correlate with cognitive deficit in Alzheimer’s disease patients and lowering of osteopontin may predict cognitive improvement.
Participants treated with TPN-101 for the entire 48-week trial duration showed a stabilization of their clinical symptoms as measured by the PSP Rating Scale (PSPRS) between weeks 24 and 48. In contrast, participants who had been on placebo from weeks 1 to 24 continued to show a worsening of the PSPRS between weeks 24 and 48, suggesting a delay of clinical benefit onset of at least 24 weeks after start of drug treatment, and lagging behind the early effects on biomarkers seen in weeks 1 to 24.
“There are no effective treatments for PSP, a uniformly fatal disease that is similarly prevalent to ALS,” said Adam Boxer, M.D., Ph.D., Endowed Professor in Memory and Aging in the Department of Neurology at the University of California, San Francisco and principal investigator in the Phase 2 study of TPN-101 for PSP. “The effects of TPN-101 on CSF NfL concentrations, as well as other exploratory CSF biomarkers, have not previously been observed in any PSP trial and support further investigation of clinical treatment effects in a larger study.”
Interim Phase 2 C9orf72-Related ALS/FTD Study Results
Results from the pre-defined interim analysis of the Phase 2 study in C9orf72-related ALS/FTD demonstrated the impact of TPN-101 treatment on key biomarkers of neurodegeneration, neuroinflammation, and microglial activation including NfL, tau, UCHL1, YKL-40, and osteopontin from baseline to 24 weeks. The data also showed a clinical effect on Vital Capacity, an objective respiratory measure that correlates with mortality in patients with ALS. Notably, C9orf72-related ALS/FTD is a TDP-43 protein pathology associated with nuclear displacement of TDP-43 in the central nervous system. TDP-43 pathology also occurs in about 50% of patients with Alzheimer’s disease.
“Transposon’s founding mission was to establish human proof-of-concept that dysregulation of retrotransposable elements in neurodegenerative diseases such as PSP, ALS and Alzheimer’s disease can be addressed with a new class of LINE-1 reverse transcriptase inhibitors,” stated Eckard Weber, M.D., Founder and Chief Innovation Officer of Transposon. “The results obtained with TPN-101 in both PSP and ALS/FTD show for the first time that neurodegenerative diseases that involve LINE-1 dysregulation are treatable with a specific inhibitor of this important novel target. We look forward to advancing the development of TPN-101 into registration studies to address this devastating and rapidly growing disease category.”
“Given the large unmet needs in PSP and ALS, these promising results highlight the therapeutic potential of TPN-101 for both disorders,” added Murali Doraiswamy, MBBS, FRCP, Professor of Psychiatry and Geriatrics at Duke University and chair of Transposon’s clinical advisory board. “These data also provide key validation of targeting LINE-1 to treat other tauopathies such as Alzheimer’s disease.”
“Based on the groundbreaking study results shown by TPN-101 in both PSP and ALS/FTD, we plan to rapidly advance TPN-101 into a Phase 3 registration study for the treatment of PSP, and potentially ALS/FTD as well, pending final confirmatory study results,” said Dennis Podlesak, Chairman and Chief Executive Officer of Transposon. “Importantly, the totality of the results across both studies also provides compelling scientific rationale for targeting LINE-1 to treat Alzheimer’s disease. Based on these findings, we are expanding our development efforts into Alzheimer’s disease with the goal of bringing a novel drug treatment to patients that remain in dire need of an effective new therapeutic option.”
About AD/PD 2024
The hybrid AD/PD 2024: 18th International Conference on Alzheimer’s and Parkinson’s Diseases will take place online and in Lisbon, Portugal, from March 5-9, 2024. Transposon will present final results from its Phase 2 study in PSP in a poster presentation (abstract #433) at AD/PD 2024 entitled: “A Phase 2a Study of TPN-101, a Nucleoside Reverse Transcriptase Inhibitor, in Patients with Progressive Supranuclear Palsy.” For more information, please visit the AD/PD 2024 website.
About the Phase 2 Study in PSP
The Phase 2 study in PSP is a multi-center, randomized, double-blind, placebo-controlled, parallel-group, 4-arm study with an open-label treatment phase in patients with PSP. Participants (n=42) were randomized to receive daily doses of 100 mg, 200 mg or 400 mg of TPN-101, or placebo. The study includes a 6-week screening period, a 24-week double-blind treatment period, a 24-week open label treatment period, and a follow-up visit 4 weeks post-treatment. All phases of the study, including the 24-week open label treatment period, have been completed. Further information on the study can be accessed at ClinicalTrials.gov.
About the Phase 2 Study in C9orf72-related ALS/FTD
The Phase 2 study in C9orf72-related ALS/FTD is multi-center, randomized, double-blind, placebo-controlled parallel-group, 2-arm study with an open-label treatment phase in patients with C9orf72-related ALS and/or FTD. Participants (n=42) were randomized to receive daily doses of 400 mg of TPN-101 or placebo. Similar to the Phase 2 study in PSP, this study includes a 6-week screening period, a 24-week double-blind treatment period, a 24-week open-label treatment period, and a follow-up visit 4 weeks post-treatment. The predefined interim analysis was performed after all patients completed the 24-week double-blind portion of the study. The open-label extension of the study is ongoing, with subjects and investigators blinded to the original treatment assignment. Further information on the study can be accessed at ClinicalTrials.gov.
About TPN-101
TPN-101 specifically inhibits the LINE-1 reverse transcriptase that promotes LINE-1 replication. LINE-1 elements are a class of retrotransposable elements that in humans are uniquely capable of replicating and moving to new locations within the genome. When this process becomes dysregulated, LINE-1 reverse transcriptase drives overproduction of LINE-1 DNA, triggering innate immune responses that contribute to neurodegenerative, autoimmune and aging-related disease pathology.
About PSP
PSP is a rare and fatal tauopathy with no approved treatment options. PSP mainly affects people in their mid- to late-60s and is characterized by early postural instability and falls, vertical gaze palsy, akinesia, rigidity, pseudobulbar palsy, and frontal dysfunction with cognitive and behavioral changes. The mean survival for individuals with PSP is 6 to 7 years.
About ALS and FTD
ALS is a neurodegenerative disease characterized by progressive muscle weakness, loss of ability to speak, eat, move or breathe. FTD is a progressive frontal / temporal cortex disease associated with behavior and personality changes, emotional problems, and difficulty walking, communicating or working. With onset commonly in middle age or earlier, patients with ALS have a mean survival of only 3 years. Patients with FTD have a mean survival of 9 years.
About Transposon
Transposon Therapeutics, Inc. is a clinical-stage biopharmaceutical company developing a platform of novel therapies for the treatment of neurodegenerative and aging-related diseases, including Alzheimer’s disease. The company’s lead clinical compound, TPN-101, is first-in-class to address LINE-1 reverse transcriptase for treating neurodegenerative and autoimmune diseases. The company also has a discovery platform supporting a deep pipeline of novel therapies to address additional indications. Dr. Doraiswamy chairs the company’s clinical advisory board and has received compensation/stock options for his role.
Contact:
Rick Orr
Transposon Therapeutics, Inc.
(858) 535-4821
rorr@transposonrx.com
SOURCE Transposon Therapeutics
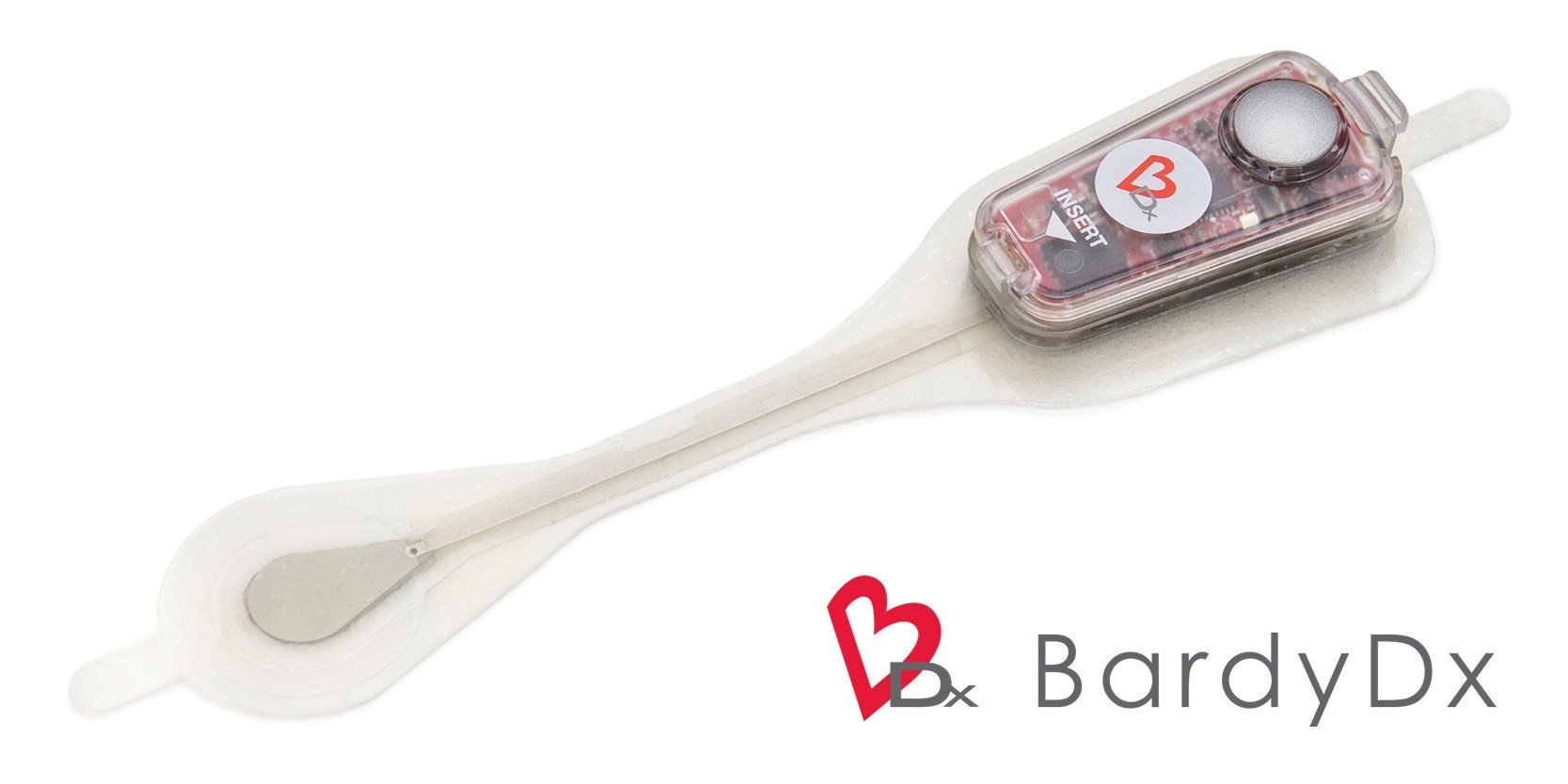 The Carnation Ambulatory Monitor (CAM™) is the first non-invasive, P-wave centric ambulatory cardiac monitor and arrhythmia detection device designed to elevate signal detection, patient compliance, and physician care procedures. Through advanced compression algorithms to more effectively process signals, and short patch vectors to overcome signal-to-noise limitations, the technology is rigorously optimized to ensure a clear P-wave recording and superior detection of heart rhythms. The form and placement of the device are designed to improve comfort for patients, while generating higher rhythm specificity. The sensor is placed over the sternum, on top of the heart, this location prevents any noisy muscle stimulation and enhances the capture of the P-wave signal.
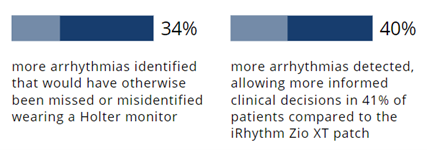
The Carnation Ambulatory Monitor (CAM™) is the first non-invasive, P-wave centric ambulatory cardiac monitor and arrhythmia detection device designed to elevate signal detection, patient compliance, and physician care procedures. Through advanced compression algorithms to more effectively process signals, and short patch vectors to overcome signal-to-noise limitations, the technology is rigorously optimized to ensure a clear P-wave recording and superior detection of heart rhythms. The form and placement of the device are designed to improve comfort for patients, while generating higher rhythm specificity. The sensor is placed over the sternum, on top of the heart, this location prevents any noisy muscle stimulation and enhances the capture of the P-wave signal. underserved needs of comfort for the female chest. With a lifestyle-enabling form factor patients can remain going about their daily activities comfortably and confidently. CAM™ provides options for a 48-hour, 7- or 14-day wear period and continuous 24/7 monitoring using BDxCONNECT, a versatile patient management portal. When experiencing symptoms, patients simply push the button on the device, and a report is sent to their physician to analyze and determine if intervention is needed. Once the wear period is finished, the data is uploaded and a more in-depth view of ECG data is generated through multiple fields of concise analysis. In an industry-leading two-day report turn-around, physicians have an improved resolution of ECG data, supplying more heart rhythm information and allowing for the detection of many unrecognized patterns and more clinically-actionable diagnoses.
underserved needs of comfort for the female chest. With a lifestyle-enabling form factor patients can remain going about their daily activities comfortably and confidently. CAM™ provides options for a 48-hour, 7- or 14-day wear period and continuous 24/7 monitoring using BDxCONNECT, a versatile patient management portal. When experiencing symptoms, patients simply push the button on the device, and a report is sent to their physician to analyze and determine if intervention is needed. Once the wear period is finished, the data is uploaded and a more in-depth view of ECG data is generated through multiple fields of concise analysis. In an industry-leading two-day report turn-around, physicians have an improved resolution of ECG data, supplying more heart rhythm information and allowing for the detection of many unrecognized patterns and more clinically-actionable diagnoses.
![]()
 Cognitive deficits are the hallmark of a variety of psychiatric and neurodegenerative disorders affecting millions of patients worldwide. These conditions include; major depressive disorder (MDD), schizophrenia, a number of neurodegenerative disorders including Alzheimer’s Disease, and aging in general. Treatment options are few and far between and in many cases no treatment options are available to address cognitive deficits across disorders which often leave patients, care givers and family members struggling. At the forefront of research in the area is Dr Etienne Sibille and his lab at the Centre for Mental Health and Addiction (CAMH) at the University of Toronto, a world leading academic research institute. Dr. Sibille and his team has been studying how brain circuitry is affected and if there are ways in which the connections between neurons in vital areas responsible for cognition can be strengthened and as we age.
Cognitive deficits are the hallmark of a variety of psychiatric and neurodegenerative disorders affecting millions of patients worldwide. These conditions include; major depressive disorder (MDD), schizophrenia, a number of neurodegenerative disorders including Alzheimer’s Disease, and aging in general. Treatment options are few and far between and in many cases no treatment options are available to address cognitive deficits across disorders which often leave patients, care givers and family members struggling. At the forefront of research in the area is Dr Etienne Sibille and his lab at the Centre for Mental Health and Addiction (CAMH) at the University of Toronto, a world leading academic research institute. Dr. Sibille and his team has been studying how brain circuitry is affected and if there are ways in which the connections between neurons in vital areas responsible for cognition can be strengthened and as we age. Dr. Sibille’s group embarked on a journey to design a drug that could reverse the pathology that they discovered and hypothesized was leading to cognitive decline in patients. By the summer of 2017, when we first met Dr. Sibille and his associate Dr. Prevost, — they, CAMH and international collaborators had filed patents on a class of compounds that showed promise in laboratory studies to address this issue. It was easy for us to be engaged by the vision, need and opportunity. While the team had initially identified a group of novel compounds and very interesting biology, translating that into a drug was still far off. From 2017 to 2021, through hard work, tenacity, constant collaboration with other drug developers in the field, leveraging connections through various local and international accelerator programs, VC’s like us, clinicians, etc. the team was able to identify a lead drug that had all the characteristics needed. They also learned the art of approaching and working with investors, contract organizations that do much of the preclinical work, the various regulatory bodies like FDA, and clinicians experienced running clinical studies. Through this process the company settled on a program to first address the huge unmet clinical need in patients that respond to treatment of MDD using antid epressants but continue to have lingering cognitive issues. It is estimated that in the US and Europe alone that there are more than 4.3 million suffering from cognitive impairment that have been successfully treated for MDD. Therapeutic strategies currently available today include expensive psychotherapy, cognitive remediation and more recently transcranial magnetic stimulation and currently there are no approved therapeutics to treat the cognitive symptoms.
Dr. Sibille’s group embarked on a journey to design a drug that could reverse the pathology that they discovered and hypothesized was leading to cognitive decline in patients. By the summer of 2017, when we first met Dr. Sibille and his associate Dr. Prevost, — they, CAMH and international collaborators had filed patents on a class of compounds that showed promise in laboratory studies to address this issue. It was easy for us to be engaged by the vision, need and opportunity. While the team had initially identified a group of novel compounds and very interesting biology, translating that into a drug was still far off. From 2017 to 2021, through hard work, tenacity, constant collaboration with other drug developers in the field, leveraging connections through various local and international accelerator programs, VC’s like us, clinicians, etc. the team was able to identify a lead drug that had all the characteristics needed. They also learned the art of approaching and working with investors, contract organizations that do much of the preclinical work, the various regulatory bodies like FDA, and clinicians experienced running clinical studies. Through this process the company settled on a program to first address the huge unmet clinical need in patients that respond to treatment of MDD using antid epressants but continue to have lingering cognitive issues. It is estimated that in the US and Europe alone that there are more than 4.3 million suffering from cognitive impairment that have been successfully treated for MDD. Therapeutic strategies currently available today include expensive psychotherapy, cognitive remediation and more recently transcranial magnetic stimulation and currently there are no approved therapeutics to treat the cognitive symptoms.