Dissident Withdraws the Consents of its Nominees from Consideration Prior to the AGM
Aurinia Reaffirms its Commitment to Voclosporin and Creating Shareholder Value
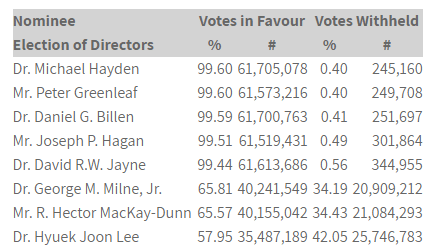
VICTORIA, British Columbia–(BUSINESS WIRE)– Aurinia Pharmaceuticals Inc. (NASDAQ:AUPH / TSX:AUP) (“Aurinia” or the “Company”) is pleased to announce that shareholders of the Company (the “Shareholders”) elected all eight of the Company’s highly qualified director nominees at the Company’s Annual General Meeting held on June 26, 2019 (the “Meeting”).
The Dissident shareholder, ILJIN SNT Co., LTD (“ILJIN”), provided notice to withdraw the consents of its director nominees prior to the Meeting after having witnessed the strong proxy results in favour of management’s nominees to Aurinia’s Board.
“We appreciate the strong and unequivocal support from our shareholders,” said Aurinia’s Chairman of the Board, Dr. George M. Milne, Jr. “The Board and management greatly value the numerous conversations we have had with our shareholders over the last few months regarding our ongoing commitment to drive shareholder value through the advancement of and preparation for commercialization of voclosporin to address the unmet medical needs of patients around the globe. Further, we will continue to maintain an active and productive dialogue with Aurinia’s investor community as we continue to execute on our strategy.”
Shareholders also approved the non-binding advisory “say-on-pay” resolution approving the Company’s approach to executive compensation with 64.31% voting in favour and 35.69% voting against. Excluding the 1,920,455 common shares known to be owned or controlled by directors, officers, employees and consultants of the Company and the 13,716,567 common shares known to be owned or controlled by ILJIN from the tally on this resolution, approximately 81.82% of common shares were voted in favour of the advisory resolution on executive compensation.
All other matters voted on at the Meeting, which included confirming the number of directors at eight and approving the reappointment of PricewaterhouseCoopers LLP as auditors, were also approved. Voting results on all matters voted on at the Meeting will be filed on SEDAR and EDGAR.
About Aurinia
Aurinia Pharmaceuticals is a late clinical-stage biopharmaceutical company focused on developing and commercializing therapies to treat targeted patient populations that are impacted by serious diseases with a high unmet medical need. The Company is currently developing an investigational drug, for the treatment of Lupus Nephritis, Focal Segmental Glomerulosclerosis and Dry Eye Syndrome. The Company’s head office is in Victoria, British Columbia and focuses its development efforts globally.
About Voclosporin
Voclosporin, an investigational drug, is a novel and potentially best-in-class calcineurin inhibitor (“CNI”) with clinical data in over 2,600 patients across indications. Voclosporin is an immunosuppressant, with a synergistic and dual mechanism of action. By inhibiting calcineurin, voclosporin blocks IL-2 expression and T-cell mediated immune responses and stabilizes the podocyte in the kidney. It has been shown to have a more predictable pharmacokinetic and pharmacodynamic relationship (potentially requires no therapeutic drug monitoring), an increase in potency (vs cyclosporin), and an improved metabolic profile compared to legacy CNIs. Aurinia anticipates that upon regulatory approval, patent protection for voclosporin will be extended in the United States and certain other major markets, including Europe and Japan, until at least October 2027 under the Hatch-Waxman Act and comparable laws in other countries and until April 2028 with anticipated pediatric extension. Further, the new Notice of Allowance is expected to result in the issuance of a U.S. patent with a term extending to December 2037. If the FDA approves the use of voclosporin for LN and the label for such use follows the dosing protocol under the Notice of Allowance, the issuance of this patent will expand the scope of intellectual property protection for voclosporin to December 2037.
About VOS
Voclosporin ophthalmic solution (“VOS”) is an aqueous, preservative free nanomicellar solution intended for use in the treatment of DES. A Phase 2a study was recently completed with results released in January of 2019. Previously, a Phase 1 study with healthy volunteers and patients with DES was also completed as were studies in rabbit and dog models. VOS has IP protection until 2031.
Forward-Looking Statements
Certain statements made in this press release may constitute forward-looking information within the meaning of applicable Canadian securities law and forward-looking statements within the meaning of applicable United States securities law. These forward-looking statements or information include but are not limited to statements or information with respect to: Aurinia’s anticipation that upon regulatory approval, patent protection for voclosporin will be extended in the United States and certain other major markets, including Europe and Japan, until at least October 2027 under the Hatch-Waxman Act and comparable laws in other countries and until April 2028 with anticipated pediatric extension; that the new Notice of Allowance is expected to result in the issuance of a U.S. patent with a term extending to December 2037; that if the FDA approves the use of voclosporin for LN and the label for such use follows the dosing protocol under the Notice of Allowance, the issuance of this patent will expand the scope of intellectual property protection for voclosporin to December 2037; Aurinia’s ongoing commitment to drive shareholder value through the advancement and commercialization of voclosporin and to maintain an active dialogue with its investment community as it continues to execute on the Company’s strategy.
It is possible that such results or conclusions may change based on further analyses of these data. Words such as “anticipate”, “will”, “believe”, “estimate”, “expect”, “intend”, “target”, “plan”, “goals”, “objectives”, “may” and other similar words and expressions, identify forward-looking statements. We have made numerous assumptions about the forward-looking statements and information contained herein, including among other things, assumptions about: Aurinia being able to extend and protect its patents on terms acceptable to Aurinia, Aurinia successfully completing its clinical trials, Aurinia receiving regulatory approval on terms acceptable to Aurinia, and Aurinia having sufficient funds on hand to complete its trials and operations as currently planned.
Even though the management of Aurinia believes that the assumptions made, and the expectations represented by such statements or information are reasonable, there can be no assurance that the forward-looking information will prove to be accurate.
Forward-looking information by their nature are based on assumptions and involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of Aurinia to be materially different from any future results, performance or achievements expressed or implied by such forward-looking information. Should one or more of these risks and uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in forward-looking statements or information. Such risks, uncertainties and other factors include, among others, the following: Aurinia not being able to extend or fully protect its patent portfolio for voclosporin, Aurinia not obtaining necessary regulatory approval, negative results from clinical trials, and cash outlays being higher than currently planned.
Although we have attempted to identify factors that would cause actual actions, events or results to differ materially from those described in forward-looking statements and information, there may be other factors that cause actual results, performances, achievements or events to not be as anticipated, estimated or intended. Also, many of the factors are beyond our control. There can be no assurance that forward-looking statements or information will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, you should not place undue reliance on forward-looking statements or information.
Except as required by law, Aurinia will not update forward-looking information. All forward-looking information contained in this press release is qualified by this cautionary statement. Additional information related to Aurinia, including a detailed list of the risks and uncertainties affecting Aurinia and its business can be found in Aurinia’s most recent Annual Information Form available by accessing the Canadian Securities Administrators’ System for Electronic Document Analysis and Retrieval (SEDAR) website at www.sedar.com or the U.S. Securities and Exchange Commission’s Electronic Document Gathering and Retrieval System (EDGAR) website at www.sec.gov/edgar.
Company Contact:
Glenn Schulman, PharmD, MPH
Corporate Communications
gschulman@auriniapharma.com
Shareholder Questions:
Laurel Hill Advisory Group
North American Toll Free: 1-877-452-7184
Collect Calls Outside North America:
1-416-304-0211
assistance@laurelhill.com
Related Article: Aurinia Further Strengthens Its Board of Directors with the Appointment of Dr. Daniel Billen