VICTORIA, British Columbia–(BUSINESS WIRE)– Aurinia Pharmaceuticals Inc. (NASDAQ: AUPH / TSX:AUP) (“Aurinia” or the “Company”) today reported financial results for the first quarter ended March 31, 2019, and provided an update on recent operational highlights. Amounts, unless specified otherwise, are expressed in U.S. dollars.
First Quarter 2019 Highlights
- Fully-enrolled AURORA Phase 3 trial in lupus nephritis (“LN”) continues on track with results anticipated in late 2019.
- Reported results from a Phase 2a Dry Eye study with voclosporin ophthalmic solution (“VOS”) that achieved statistically superior efficacy in secondary objective endpoints compared to cyclosporin ophthalmic emulsion 0.05% (Restasis®), the current DES market leader. VOS did not meet the primary endpoint as both drugs were well tolerated and demonstrated less than anticipated drop discomfort.
- Received a Notice of Allowance from the United States Patent and Trademark Office (“USPTO”) for claims which have the potential to cover voclosporin’s method of use and dosing protocol for lupus nephritis (“LN’) until December 2037.
- Appointed Mr. Peter Greenleaf as Chief Executive Officer and Dr. George Milne to Chairman of the Board of Directors.
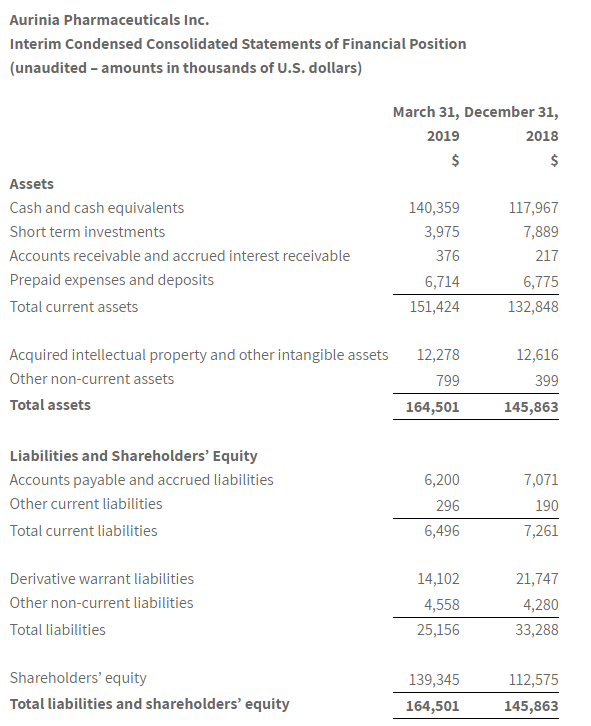
- Cash, cash equivalents, and short-term investments of $144.3 million as of March 31, 2019.
Recent Director and Officer Appointments
On April 29, 2019, Aurinia appointed Peter Greenleaf as Chief Executive Officer and a Director on the Aurinia Board.
Concurrently, Dr. Richard M. Glickman, who previously announced his plans to retire on November 6, 2018, stepped down from his role as Chairman and CEO. Dr. Glickman remains an advisor to the Company for a period of 12 months.
“It is an honor to join Aurinia at this time and lead the organization through its next phase of growth to advance voclosporin toward commercialization based upon the Phase 3 AURORA results in lupus nephritis anticipated by the end of this year,” commented Mr. Peter Greenleaf, Chief Executive Officer of Aurinia. “After following the Aurinia story and after conducting further due diligence, I am truly impressed with the Aurinia team, their ability to execute and advance voclosporin in a cost-efficient manner, with the ongoing goal of bringing voclosporin to help patients suffering from LN.”
In conjunction with Dr. Glickman’s retirement as the Chairman, the Board elevated George M. Milne, Jr., Ph.D., to the position of Chairman of the Board effective April 29, 2019. In addition, the Board appointed Dr. Daniel Billen to the Board also effective April 29, 2019.
Dr. Milne stated, “With the appointment of Peter and Daniel to the board, combined with our experienced and committed employees and management team, Aurinia is strongly positioned to achieve our milestones and maximize the value of voclosporin for all of our stakeholders.
VOS for Dry Eye Syndrome (“DES”)
Based upon the exploratory Phase 2a results generated with VOS in a head-to-head comparison vs. the current market leader for the treatment of DES, Aurinia plans to initiate a Phase 2/3 study by late 2019. This study will encompass certain critical regulatory requirements that the FDA has traditionally required for DES product approval, these requirements include both dose-optimization requirements along with a comparison versus vehicle.
“I’m confident that the internal Aurinia team along with our key ophthalmology clinical advisors have crafted a framework of a plan that minimizes the clinical and regulatory risk for VOS and maximizes our probability of launching VOS into the multi-billion dollar DES market in due course,” said Michael R. Martin, Chief Operating Officer of Aurinia.
Financial Liquidity at March 31, 2019
As at March 31, 2019, Aurinia had cash, cash equivalents and short-term investments of $144.3 million compared to $125.9 million of cash, cash equivalents and short-term investments as at December 31, 2018. Net cash used in operating activities was $13.1 million for the first quarter ended March 31, 2019, compared to $14.4 million for the first quarter ended March 31, 2018.
The Company believes, that based on its current plans that Aurinia has sufficient financial resources to fund the existing LN program, including the AURORA trial and the AURORA 2 extension trial, complete the NDA submission to the FDA, conduct the ongoing Phase 2 study for FSGS, commence additional DES studies and fund operations into mid-2020.
The increase in our cash position at March 31, 2019, was primarily the result of the following:
At-The-Market (“ATM”) Facility
On November 30, 2018, Aurinia had entered into an open market sale agreement with Jefferies LLC pursuant to which the Company could from time to time sell, through ATM offerings, common shares that would have an aggregate offering amount of up to $30 million. The ATM was fully utilized in the first quarter. Aurinia received gross proceeds of $30 million and issued 4.6 million common shares. The Company incurred share issue costs of $1.2 million including a 3% commission and professional and filing fees related to the ATM offerings.
February 14, 2014 Warrant Exercises
The remaining derivative warrants outstanding from the February 14, 2014, private placement were exercised in the first quarter ended March 31, 2019. Certain holders of these warrants elected the cashless exercise option and the Company issued 687,000 common shares on the cashless exercise of 1.3 million warrants. Three holders of 464,000 warrants exercised these warrants for cash, at a price of $3.2204 per common share. The Company received cash proceeds of $1.5 million and issued 464,000 common shares.
Financial Results for the First Quarter Ended March 31, 2019
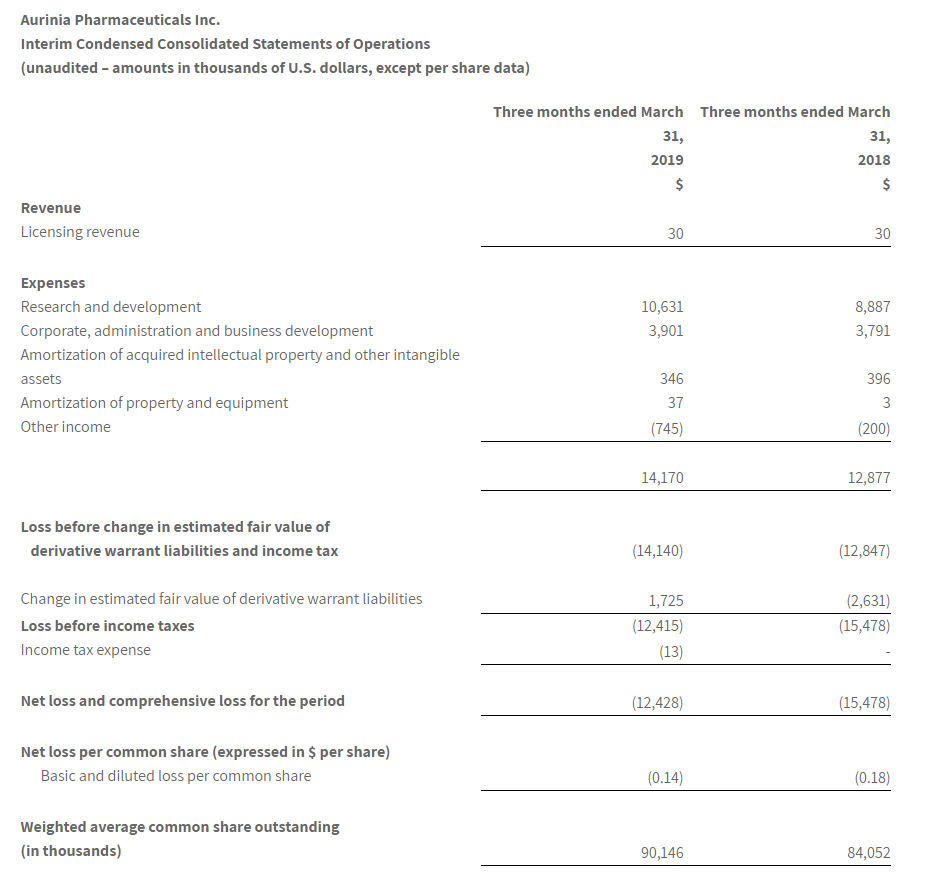
The Company reported a consolidated net loss of $12.4 million or $0.14 per common share for the first quarter ended March 31, 2019, as compared to a consolidated net loss of $15.5 million or $0.18 per common share for the first quarter ended March 31, 2018.
The loss for the first quarter ended March 31, 2019, reflected a reduction of $1.7 million in the estimated fair value of derivative warrant liabilities compared to an increase of $2.6 million in the estimated fair value of derivative warrant liabilities for the first quarter ended March 31, 2018. The derivative warrant liabilities will ultimately be eliminated on the exercise or forfeiture of the warrants and will not result in any cash outlay by the Company.
The loss before the change in estimated fair value of derivative warrant liabilities and income tax was $14.1 million for the first quarter ended March 31, 2019, compared to $12.9 million for the same period in 2018.
Research and development (“R&D”) expenses increased to $10.6 million for the first quarter ended March 31, 2019, compared to $8.9 million for the first quarter ended March 31, 2018. The increase in these expenses primarily reflected completion costs for the DES study and higher costs incurred for the AURORA 2 extension trial, the DDI study and the FSGS Phase 2a study as these studies had more activity in the first quarter ended March 31, 2019, compared to the same period in 2018.
Corporate, administration and business development expenses increased slightly to $3.9 million for the first quarter of 2019, compared to $3.8 million for the first quarter of 2018.
This press release should be read in conjunction with our unaudited interim condensed consolidated financial statements and the Management’s Discussion and Analysis for the first quarter ended March 31, 2019, which are accessible on Aurinia’s website, on SEDAR or on EDGAR.
Aurinia will host a conference call and webcast to discuss the first quarter ended March 31, 2019, financial results today, Monday, May 13, 2019, at 4:30 p.m. ET. This event can be accessed on the investor section of the Aurinia website.
About Aurinia
Aurinia Pharmaceuticals is a late clinical-stage biopharmaceutical company focused on developing and commercializing therapies to treat targeted patient populations that are impacted by serious diseases with a high unmet medical need. The Company is currently developing an investigational drug, for the treatment of Lupus Nephritis, Focal Segmental Glomerulosclerosis, and Dry Eye Syndrome. The Company’s head office is in Victoria, British Columbia and focuses its development efforts globally.
About Voclosporin
Voclosporin, an investigational drug, is a novel and potentially best-in-class calcineurin inhibitor (“CNI”) with clinical data in over 2,600 patients across indications. Voclosporin is an immunosuppressant, with a synergistic and dual mechanism of action. By inhibiting calcineurin, voclosporin blocks IL-2 expression and T-cell mediated immune responses and stabilizes the podocyte in the kidney. It has been shown to have a more predictable pharmacokinetic and pharmacodynamic relationship (potentially requires no therapeutic drug monitoring), an increase in potency (vs cyclosporin), and an improved metabolic profile compared to legacy CNIs. Aurinia anticipates that upon regulatory approval, patent protection for voclosporin will be extended in the United States and certain other major markets, including Europe and Japan, until at least October 2027 under the Hatch-Waxman Act and comparable laws in other countries and until April 2028 with anticipated pediatric extension. Further, the new Notice of Allowance is expected to result in the issuance of a U.S. patent with a term extending to December 2037. If the FDA approves the use of voclosporin for LN and the label for such use follows the dosing protocol under the Notice of Allowance, the issuance of this patent will expand the scope of intellectual property protection for voclosporin to December 2037.
About VOS
Voclosporin ophthalmic solution (“VOS”) is an aqueous, preservative-free nanomicellar solution intended for use in the treatment of DES. A Phase 2a study was recently completed with results released in January of 2019. Previously, a Phase 1 study with healthy volunteers and patients with DES was also completed as were studies in rabbit and dog models. VOS has IP protection until 2031.
About LN
Lupus Nephritis (“LN”) in an inflammation of the kidney caused by Systemic Lupus Erythematosus (“SLE”) and represents a serious progression of SLE. SLE is a chronic, complex and often disabling disorder. The disease is highly heterogeneous, affecting a wide range of organs and tissue systems. Unlike SLE, LN has straightforward disease outcomes (measuring proteinuria) where an early response correlates with long-term outcomes. In patients with LN, renal damage results in proteinuria and/or hematuria and a decrease in renal function as evidenced by reduced estimated glomerular filtration rate (“eGFR”), and increased serum creatinine levels. LN is debilitating and costly and if poorly controlled, LN can lead to permanent and irreversible tissue damage within the kidney, resulting in end-stage renal disease (“ESRD”), thus making LN a serious and potentially life-threatening condition.
About FSGS
Focal segmental glomerulosclerosis (“FSGS”) is a rare disease that attacks the kidney’s filtering units (glomeruli) causing serious scarring which leads to permanent kidney damage and even renal failure. FSGS is one of the leading causes of Nephrotic Syndrome (“NS”) and is identified by biopsy and proteinuria. NS is a collection of signs and symptoms that indicate kidney damage, including large amounts of protein in the urine; low levels of albumin and higher than normal fat and cholesterol levels in the blood, and edema. Similar to LN, early clinical response (measured by reduction of proteinuria) is thought to be critical to long-term kidney health in patients with FSGS. Currently, there are no approved therapies for FSGS in the United States and the European Union.
About DES
Dry eye syndrome (“DES”) is characterized by irritation and inflammation that occurs when the eye’s tear film is compromised by reduced tear production, imbalanced tear composition, or excessive tear evaporation. The impact of DES ranges from subtle, yet constant eye irritation to significant inflammation and scarring of the eye’s surface. Discomfort and pain resulting from DES can reduce quality of life and cause difficulty reading, driving, using computers and performing daily activities. DES is a chronic disease. There are currently three FDA approved therapies for the treatment of dry eye; however, there is opportunity for potential improvement in the effectiveness by enhancing tolerability, onset of action and alleviating the need for repetitive dosing.
Forward-Looking Statements
Certain statements made in this press release may constitute forward-looking information within the meaning of applicable Canadian securities law and forward-looking statements within the meaning of applicable United States securities law. These forward-looking statements or information include but are not limited to statements or information with respect to AURORA having data around the end of this year, completing NDA submissions in a successful and timely manner, voclosporin being potentially a best-in-class CNI with robust intellectual property exclusivity; and that Aurinia has sufficient financial resources to fund the existing LN program, including the AURORA trial, and the NDA submission to the FDA, conduct the current Phase 2a study for FSGS, commence additional studies for DES and fund operations into mid-2020 and that the efficacy endpoint clearly signals that VOS has the potential to have a more rapid onset than Restasis® as measured by signs of the disease. It is possible that such results or conclusions may change based on further analyses of these data. Words such as “anticipate”, “will”, “believe”, “estimate”, “expect”, “intend”, “target”, “plan”, “goals”, “objectives”, “may” and other similar words and expressions, identify forward-looking statements. We have made numerous assumptions about the forward-looking statements and information contained herein, including among other things, assumptions about: the market value for the LN & DES programs; that another company will not create a substantial competitive product for Aurinia’s LN and DES business without violating Aurinia’s intellectual property rights; the burn rate of Aurinia’s cash for operations; the costs and expenses associated with Aurinia’s clinical trials; the planned studies achieving positive results; Aurinia being able to extend and protect its patents on terms acceptable to Aurinia; and the size of the LN or DES markets. Even though the management of Aurinia believes that the assumptions made, and the expectations represented by such statements or information are reasonable, there can be no assurance that the forward-looking information will prove to be accurate.
Forward-looking information by their nature are based on assumptions and involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of Aurinia to be materially different from any future results, performance or achievements expressed or implied by such forward-looking information. Should one or more of these risks and uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described in forward-looking statements or information. Such risks, uncertainties and other factors include, among others, the following: difficulties, delays, or failures we may experience in the conduct of our AURORA clinical trial; difficulties we may experience in completing the development and commercialization of voclosporin; the market for the LN business may not be as estimated; Aurinia may have to pay unanticipated expenses; estimated costs for clinical trials may be underestimated, resulting in Aurinia having to make additional expenditures to achieve its current goals; Aurinia not being able to extend or fully protect its patent portfolio for voclosporin; and competitors may arise with similar products. Although we have attempted to identify factors that would cause actual actions, events or results to differ materially from those described in forward-looking statements and information, there may be other factors that cause actual results, performances, achievements or events to not be as anticipated, estimated or intended. Also, many of the factors are beyond our control. There can be no assurance that forward-looking statements or information will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, you should not place undue reliance on forward-looking statements or information.
Except as required by law, Aurinia will not update forward-looking information. All forward-looking information contained in this press release is qualified by this cautionary statement. Additional information related to Aurinia, including a detailed list of the risks and uncertainties affecting Aurinia and its business can be found in Aurinia’s most recent Annual Information Form available by accessing the Canadian Securities Administrators’ System for Electronic Document Analysis and Retrieval (SEDAR) website or the U.S. Securities and Exchange Commission’s Electronic Document Gathering and Retrieval System (EDGAR) website.
Investor & Media Contacts:
Glenn Schulman, PharmD, MPH
Corporate Communications
gschulman@auriniapharma.com
Dennis Bourgeault
Chief Financial Officer
dbourgeault@auriniapharma.com
Related Article: Aurinia Reports Fourth Quarter and Full Year 2018 Financial Results and Operational Highlights